Corrosion Protection
|

Please click to download:
|
Wantar Engineering & Constructions Sdn Bhd is a wholly separate company forming part of the B.H. McDonald & Associates Pte Ltd Corrosion Group comprising three other Australian companies. The group commenced operation in 1975 and the company have been established in Singapore since 1990. The companies' corrosion protection activities include consultancy, the provision of materials and equipment and/or installation/supervision of remedial installations to the Oil and Gas Industries, Marine Industries, Onshore Pipelines and many others. Cathodic Protection describes an electrical procedure to protect steel structures exposed to corrosive aqueous environment, including seawater, brackish water, underground soil etc. It is a technique used to eliminate corrosion of a metal in an electrolyte by suppressing all the small localised corrosion currents from flowing out of the local anode into the electrolyte. This is done by introducing sufficient cathodic current to the metal concerned. This cathodic current negates or reverses the current generated in the corrosion cell and may be obtained either through Sacrificial Anode System or Impressed Current System. Corrosion Mechanism To understand Cathodic Protection, one must first understand the Corrosion mechanism. Metal that has been extracted from its primary ore (metal oxides or other free radicals) has a natural tendency to revert to that state under the action of oxygen and water. This action is called corrosion and the most common example is the rusting of steel. For corrosion to occur, three conditions must be present.
If the above conditions exist, at the more active metal surface (in this case we will consider freely corroding steel which is non uniform), the following reaction takes place at the more active sites: 2Fe -> 2Fe2+ + 4e- The free electrons travel through the metal path to the less active sites where the following reaction takes place: O2 + 4e- + 2 H20 -> 4 OH- This reaction is more commonly explained as current flow through the water from the anode (more active site) to the cathode (less active site). 
B.H. McDonald & Associates Pte Ltd Corrosion Group comprising three other Australian companies. The group commenced operation in 1975 and the company have been established in Singapore since 1990. The companies' corrosion protection activities include consultancy, the provision of materials and equipment and/or installation/supervision of remedial installations to the Oil and Gas Industries, Marine Industries, Onshore Pipelines and many others. Cathodic Protection describes an electrical procedure to protect steel structures exposed to corrosive aqueous environment, including seawater, brackish water, underground soil etc. It is a technique used to eliminate corrosion of a metal in an electrolyte by suppressing all the small localised corrosion currents from flowing out of the local anode into the electrolyte. This is done by introducing sufficient cathodic current to the metal concerned. This cathodic current negates or reverses the current generated in the corrosion cell and may be obtained either through Sacrificial Anode System or Impressed Current System. Corrosion Mechanism To understand Cathodic Protection, one must first understand the Corrosion mechanism. Metal that has been extracted from its primary ore (metal oxides or other free radicals) has a natural tendency to revert to that state under the action of oxygen and water. This action is called corrosion and the most common example is the rusting of steel. For corrosion to occur, three conditions must be present.
If the above conditions exist, at the more active metal surface (in this case we will consider freely corroding steel which is non uniform), the following reaction takes place at the more active sites: 2Fe -> 2Fe2+ + 4e- The free electrons travel through the metal path to the less active sites where the following reaction takes place: O2 + 4e- + 2 H20 -> 4 OH- This reaction is more commonly explained as current flow through the water from the anode (more active site) to the cathode (less active site). 
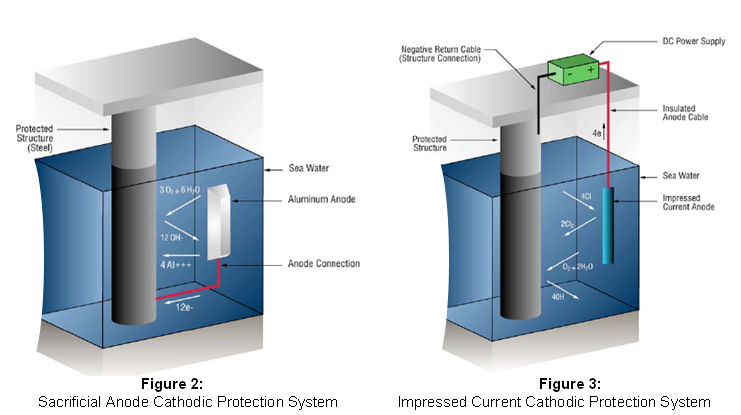
Cathodic Protection Cathodic protection prevents corrosion by converting all of the anodic (active) sites on the metal surface to cathodic (passive) sites by supplying electrical current (or free electrons) from an alternate source. Cathodic protection can be achieved in two ways:
Galvanic anode systems use galvanic anodes which are more active than steel. This practice is also referred to as a sacrificial system, since the galvanic anodes sacrifice themselves to protect the structural steel or pipeline from corrosion. The metals commonly used, as sacrificial anodes are aluminium, zinc and magnesium. Impressed-current systems use anodes of a type that are not easily dissolved into metallic ions (inert anode) and use an external source of dc power to impress a current from an external anode onto the cathode surface.
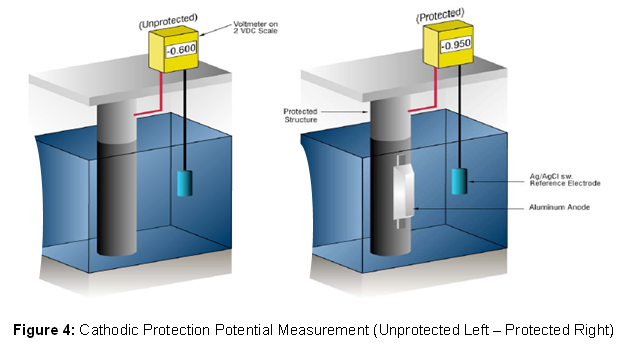
We know whether we have enough current by measuring the potential of the steel against a standard reference electrode, usually silver silver/chloride (Ag/AgCl sw.), but sometimes zinc (Zn sw.). Current flow onto any metal shifts its normal potential in the negative direction. History has shown that if steel receives enough current to shift the potential to (-) 0.800 V vs. silver/silver chloride, the corrosion is essentially stopped.
Application of Cathodic Protection: Structures that are commonly protected by Cathodic Protection are the exterior surfaces of: Underground Pipelines Ships' hulls Storage tank bases Jetties and harbour structures Steel sheet, tubular and foundation pilings Offshore platforms, floating and subsea structures Cathodic Protection is also used to protect the internal surfaces of: Large diameter pipelines Ship's tanks (product and ballast) Storage tanks (oil and water) Water-circulating systems
|